-40%
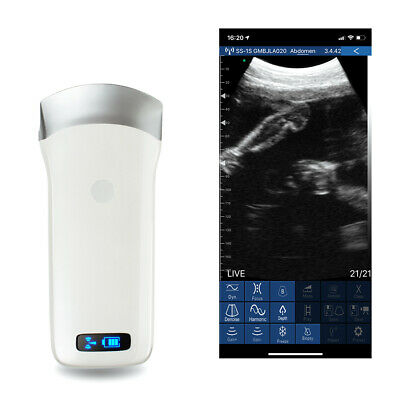
WIFI Ultrasound Scanner Convex Sensor 3.5Mhz 128 Elements Ultrasound Device Fast
$ 1029.07
- Description
- Size Guide
Description
Portable WIFI Ultrasound Scanner Convex Probe 3.5Mhz 128 Elements 32 ChannelSKU:500324
For USA Buyer: ship from USA warehouse . Fast delivery (2-6 days);
For Other Buyer: ship from China Warehouse. 2-4 weeks delivery.
Description:
3.5Mhz 128 Elements 32 Channel
How it works?
“Wireless ultrasound probe” is a mini ultrasound scanner that without screen. We made the main unit condensed into a small circuit board builded-in the probe, and showing image in smart phone/tablet through Wifi transferring.
Rotate buit-in screen probe is a wireless ultrasound probe add screen, image can both show in screen and tablet.
Image transferring through internal WIFI from probe, no need external Wifi signal.
Feature:
Workable with Tablet or Smart Phone
Built-in and replaceable battery
Advanced digital imaging technology, clear image
High cost-effective
Wireless connectivity, easy to operate
Small and light , easy to carry
Applicable in emergency, clinic, outdoor and vet inspection
Intelligent terminal platform, powerful expansion functions on application, storage, communication, printing.
Specification:
Scanning mode: Electronic array
Display mode: B, B/M
Frequency: 3.5MHz
Depth: Convex 100mm~305mm
Image Adjust: Gain, Focus, Harmonic, Denoise
Puncture assist function:the function of in-plane puncture guide line, out-of-plane puncture guide line, automatic blood vessel measurement, and the enhancement function of needle point development.
Measure: Length, Area, Angle, Obstetrics
Image frame rate:19 frames / second
Battery working time: 3 hours
Dimension: 156×60×20mm
Weight: 270g
Working system: Apple iOS and Android, Windows
Standard Configuration:
Main Host(probe) 1 Unit
Internal Battery and charge cable 1 Set
Statement:
FDA Disclaimer:
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(The seller's name: Helen Lee,City:Beijing , State: Beijing, Country: China, Telephone number: 86-18796658874)
This item has been cleaned and treated according to the manufacturer's instructions.
About Warranty:
We guarantees new equipment other than accessories to be free from defects in workmanship and materials for a period of
24 months
from the date of shipment under normal use and service.
Return Policy:
1. If you have a defective item which you want to return, please contact us
within 30 days
from you receive the item.
2. We will refund the money to you when we get the return items. Or replace item for you.
GOOD NOETS : Above 200$ ,we normally send the item via express delivery,such as UPS ,DHL ,TNT ,EMS ,safe and fast,normally take 7-10 business days to worldwide.
W
e
commit ourself to do 100% customer satisfactione .Thanks very much.
1).The trade is cross-border and Airmail is the cheapest post way, so it take a long time for delivery.
2).We are not Responsible for your Customs duty .
3).We don't add taxes, VAT or other hidden charges.
We accept payment by
PayPal
. PayPal enables you to make payment quickly and securely online.
1: We only accept payment via PayPal.
2: Please pay the money within 5 days after the auction ended.
3: Please offer home or mobile phone when the item's Value exceed 100(USD,EUR,AUD,GBP)
We are the manufacturer and distributor of medical equipments for more than 10 years.If you are interested in the distribution work, please feel free to contact us.
If you have any question,please feel free to contact us
before you open case or leave us negative feedback
,we'll do our best to help you .W
e
commit ourself to do 100% customer satisfactione .
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health.